What is a chemical element?
Issue 364 | Page 37 | Published Mar 2017
Description
Contrary to current IUPAC recommendations, the chemical element X should be defined as the nucleus of the X atom. Consequently, different isotopes with their different nuclei belong to different elements, each one with its own physical and chemical properties. This view leads to the conclusion that we no longer have a periodic table of the elements, but a periodic table of isotopes instead.
More from this issue
Increasingly affordable visualisation technology brings exciting opportunities for making the invisible appear visible. This can support the...
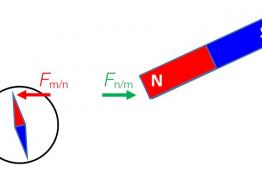
Using a conventional notation for representing forces on diagrams, students were presented with questions on the interaction between two objects....
This overview is intended to help colleagues achieve successful and satisfying observations using a ripple tank. There are many observations to...