Science note: Oxidation numbers of sulfur in the thiosulfate ion
Issue 363 | Page 10 | Published Dec 2016
Description
Oxidation numbers are the charge an atom might be imagined to have when electrons are counted according to an agreed set of rules. However, questions arise when molecules contain homopolar bonds, as these bonds are ignored when calculating oxidation numbers.
More from this issue
It is well known that schools and colleges often have budget limitations that can hamper the effectiveness of practical education. This article...
Jan 2016
Journal Article
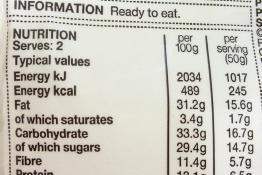
Nutrition labelling, which helps consumers to make informed choices, can be used as both a context and a vehicle for students to consolidate and...
Jan 2016
Journal Article
Tim Peake's mission to the International Space Station captured the imagination of the UK and this article describes a live radio link with...
Jan 2016
Journal Article