Science Note: Intermolecular forces
Issue 364 | Page 16 | Published Mar 2017
Description
Many students are unclear about what van der Waals and London forces are if they consult several textbooks. To clarify, van der Waals forces represent the collective term for all types of intermolecular forces, which include London forces. In general, intermolecular forces are forces of attraction between neighbouring molecules. This article outlines the different types.
More from this issue
This overview is intended to help colleagues achieve successful and satisfying observations using a ripple tank. There are many observations to...
Activation energies form an energy barrier to a chemical reaction taking place. Simple collision theory, i.e. that particles need to collide to...
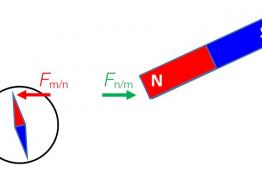
Using a conventional notation for representing forces on diagrams, students were presented with questions on the interaction between two objects....